Featured Companies
What's Next for Anthera Pharama? ANTH to focus on Kidney Failure and Renal Disease

Anthera Pharma, ANTH, Profile, Summary
Anthera Pharmaceuticals (ANTH) is a clinical-stage biopharmaceutical company focused on developing products to treat serious and life-threatening diseases, including exocrine pancreatic insufficiency and B-cell associated renal diseases.
The company was developing a Cystic Fibrosis drug called ‘Sollpura’ ,the drug was being developed in partnership with Eli Lilly, Sollpura was targeted for people who were suffering from Cystic Fibrosis which also affects pancreas and makes it much harder to digest food, the slow progression of indigestion could lead to adverse outcome including death.
Sollpurawas a ‘pure’ PIG FREE drug, unlike other drugs on the market which are derived from Pig Enzymes, Sollpurawas Kosher-Halal drug so people who prefer to avoid the consumption of drugs derived from Pigs were naturally very excited about this brand new drug.
The drug was in ‘third stage’ of clinical trials, unfortunately, the drug didn’t meet the expectation and wasn’t as effective in the late stage trials, so the company, after years of research and 100s of millions of expense, had no choice but to suspended all clinical trials of Sollpura.
The CEO released the following statement:
“We are greatly disappointed by the findings of the RESULT study,” shared
“We would like to extend our deepest gratitude to the patients and their families, study investigators, and the cystic fibrosis community for the support they have provided in the clinical development of Sollpura.”
As a result Anthera Pharmaceuticals was delisted from NASDAQ and currently trading on OTC Markets. The company has also been working on Kidney disease for a long time.
Blisibimod
Blisibimod was licensed from Amgen in December 2007 with exclusive worldwide rights. Blisibimod targets B-cell activating factor, or BAFF, which has been shown to be elevated in a variety of B-cell mediated autoimmune diseases, including Immunoglobulin A nephropathy, or IgA nephropathy, systemic lupus erythematosus, or lupus, and others.
Blisibimod is currently in development for the treatment of IgA nephropathy (IgAN). IgAN (also known as Berger’s disease) is the most common cause of primary glomerulonephritis worldwide, occurring more frequently in Asia than in Europe or North America. IgAN is characterized by deposition of IgA-anti IgA immune complexes in the kidney, resulting in inflammation, the leakage of blood and protein into the urine, and loss of kidney function. The disease typically progresses slowly but as many as 40-50% of patients will eventually develop end-stage-renal disease and require dialysis or kidney transplant. There are currently no approved therapies for IgA nephropathy
Bright • SC
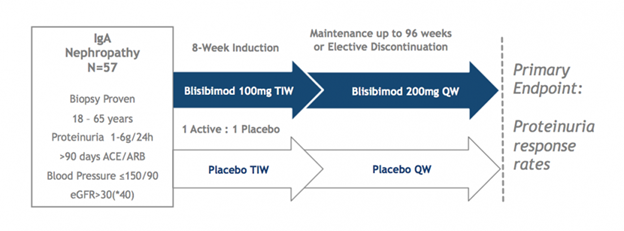
The Phase 2 BRIGHT-SC study enrolled 57 patients with biopsy-proven IgAN, 42 of whom completed at least 60 weeks of evaluation and 21 of whom completed at least 104 weeks. Two interim analyses showed favorable trends as compared to placebo on the progression of proteinuria and expected pharmacological effects (reductions in circulating B cells and serum immunoglobulins). Dosing is now completed and study results are expected in Q3 2017. Patients with persistent proteinuria (1-6 g/24hrs), despite stable background optimized therapy with angiotensin converting enzyme inhibitors (ACEi) or angiotensin receptor blockers (ARB) for at least 90 days and estimated glomerular filtration rate >30mL/min/1.73m2, were randomized to receive either blisibimod (300mg/week for 8 weeks and 200mg/week thereafter) or matching placebo for up to 104 weeks and had the option of being followed thereafter in the absence of study drug to assess longer term outcome. ACEi or ARB was continued throughout the trial as background medication. Patients were not allowed to receive corticosteroids for the treatment of IgA nephropathy within 3 months of screening.
In the most recent interim analysis, the effects of blisibimod versus placebo were assessed through at least the 48 week time point in all patients. Patients had biopsy-proven IgA nephropathy with a mean proteinuria level of 2.4 grams and an estimated glomerular filtration rate of less than 70 mL/min/1.73m2 – indicative of stage 2 chronic kidney disease per the National Kidney Foundation. A positive trend on proteinuria by blisibimod was observed. Consistent with the previously announced Week 24 analysis, blisibimod treated-patients over time demonstrated stable to slightly decreased levels of proteinuria, as assessed by urinary protein to creatinine ratio (PCR), as compared to slowly increasing levels of proteinuria in the placebo group. 44 of the original 57 patients had a Week 48 observation and 22 patients had a Week 96 observation at the time of this analysis.
Source: The Company, OxBridge Research, Daily Stock Deals, OTC Stock Wire
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!® Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
If you want to get your company featured on Daily Stock Deal or want to learn more, please contact the Editor. Thanks you!





 Penny Stock Monster
Penny Stock Monster
