Bacterin International, BONE, Profile, Summary
Bacterin International, BONE, Profile, Summary

Bacterin International Holdings (NYSE: BONE), a developer of bioactive coatings for medical applications and revolutionary bone graft material, processes and markets innovative, biologic allografts for transplantation. The Company’s biologic scaffolds, OsteoSponge®, OsteoSponge® SC and OsteoWrap®, are made from demineralized bone that is malleable and flexible, which enables more efficient and precise handling. It also markets BacFast® and OsteoLock®, which are used in spine surgery, designed to minimize graft back-out, and increase osteoinductivity. Bacterin's latest allograft, OsteoSelect® DBM Putty has excellent handling characteristics and is distributed as a sterile product, with osteoinductivity testing completed on every lot after terminal sterilization.
Bacterin’s Medical Device and Coatings Division focuses on the development of bioactive coating technologies for implantable devices. It’s core competency is anti-microbial coatings designed to reduce potential infections associated with implants, thus improving rates of healthcare-associated infections and associated costs. This Division also manages surgical kits necessary to support implantation of products processed by Bacterin’s Biologics Division.
Bacterin is an accredited tissue bank and medical device company that designs, processes, manufactures, and markets advanced medical products. Using designs focused on efficacy and safety for the patient, and functionality and ease of use for the surgeon. Bacterin is a specialty tissue bank focused on processing and distributing quality, cost-effective allografts. Bacterin operates a 32,000 sq. ft., state-of-the-art, fully compliant and FDA registered facility, equipped with five "Class 100" clean room
Sources: The Company, OxBridge Research, Daily Stock Deals, Penny Stock Monster, OTC King
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
Bone, skin, sking grafts, plastic surgery, osteo sponge, biologics, bone grafts, osteeporosis. Menopause, post menopause, Bacterin, natural medical devices.
U.S. Geothermal, HTM, Profile

U.S. Geothermal | HTM | Profile | Summary
Major Highlights
•Traded on NYSE: HTM and TSX: GTH
•Transitioned from development company to Independent Power Producer
•Proven management team
•10+years of successful project development and growth
→3 modern geothermal power plants, plus two advanced development projects
•65 MW gross generating capacity online during 2012
•Valuable, non-depleting energy from the earth
>>•Achieved positive net income, cash flow, and EBITDA
>>>2013 first year of sustainable earnings & cash flows
----->>>•Buying opportunity- Under valued stock
Vision: Building a sustainable, long-term clean
Management: Proven geothermal energy development and operating experience
Assets: Three operating geothermal power plants
•22 MW Neal Hot Springs plant Vale, Oregon
•9 MW San Emidio plant near Reno, Nevada
•13 MW Raft River plant near Pocatello,Idaho.
Advanced development properties
•El Ceibillo near Guatemala City Guatemala
•San Emidio II near Reno, Nevada
Geothermal Power Background
•Produced by utilizing heat that naturally exists within the Earth’s crust
•National Renewable Energy Lab (NREL) estimates that heat within 10,000 meters of earth’s surface is 50,000 times greater than energy that is available from petroleum and natural gas
•Geological anomalies create “shallow”reservoirs of geothermal fluids (steam and water) that can be economically exploited
•Typically reservoirs are 1,000 – 12,000 feet deep
•Geothermal fluids act as heat carriers. Those fluids are piped to the surface and used to drive turbine generators
•Geothermal fluid is reinjected to sustain reservoir pressure
•Geothermal power is renewable without significant output deterioration over time
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
Sources: US Geothermal, OxBridgeResearch, OTCking, DailyStockDeal
Geothermal power, non depleting sources, renewable sources, renewable energy labs, geothermal power plants. Sustainable, bio energy
Immnuocellular, IMUC, Profile, Summary

Immnuocellular Therapeutics | IMUC | Profile | Summary
ImmunoCellular Therapeutics is developing immune-based therapies for the treatment of brain cancer and other tumors. Our lead product candidate, ICT-107, is a dendritic cell-based vaccine targeting multiple tumor-associated antigens for glioblastoma multiforme (GBM), the most common and lethal type of brain cancer. Our pipeline also includes ICT-121, a dendritic cell (DC) vaccine targeting CD133, and ICT-140, a dendritic cell vaccine targeting ovarian cancer antigens and cancer stem cells.
ImmunoCellular Therapeutics aims to become an industry-leading, independent, commercial-stage cancer immunotherapy company serving patients in major markets.
ImmunoCellular Cancer Vaccine Manufacturing Process
Each of ImmunoCellular’s dendritic cell (DC) vaccines is prepared from blood collected from each patient. The process, which takes only a few days to complete, is designed to generate sufficient cryopreserved (frozen) cells for dosing through the induction and maintenance phases of immunotherapy. Our GMP manufacturing process adheres strictly to standard operating procedures to ensure consistency, quality and sterility.
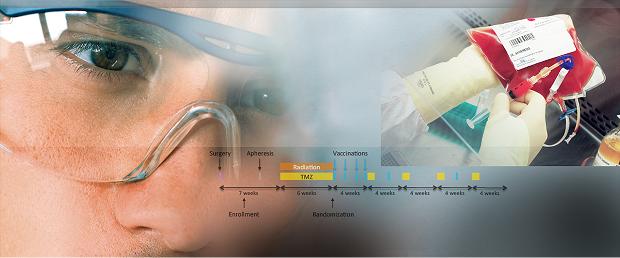
The process begins with an apheresis (also referred to as leukapheresis), a common procedure in which the patient’s blood is circulated through a machine to collect the white blood cells.
ImmunoCellular Therapeutics’ proprietary cancer vaccine immunotherapy technology has the potential to transform the treatment of cancer by improving survival and offering safer and more effective treatments than conventional cancer therapies. Our dendritic cell (DC)-based immunotherapies are designed to target both tumor cells as well as cancer stem cells by utilizing multiple tumor-specific antigens to trigger a powerful antitumor immune system response. Utilizing an advanced manufacturing process, we can produce multiple doses from a single apheresis, and offer the advantages of potency, versatility, efficiency, cost-effectiveness and convenience compared to earlier generation cancer vaccines.
Our dendritic cell (DC) vaccine technology and advanced manufacturing offer several advantages over earlier-generation cellular immunotherapies:
-
Potency
-
Matured DCs secrete IL12, which is critical for stimulating T cell killing of cancer cells.
-
More than 80% of the final manufactured product consists of matured DCs.
-
-
Versatility
-
Unlike other cell-based vaccines, our DCs target multiple antigens, thus increasing the likelihood of effective treatment.
-
-
Efficiency
-
Advanced manufacturing enables production of at least 20 doses of vaccine from a single apheresis (blood collection); thus patients do not need to undergo additional apheresis procedures.
-
-
Cost-effectiveness
-
Compared to the only approved cell-based immunotherapy, our DC vaccines can be manufactured at a significantly reduced cost.
-
-
Convenience
-
Ability to freeze and store vaccine for months or years eliminates the need to administer to the patient shortly after manufacture.
-
Intradermal administration route reduces the time and inconvenience of intravenous infusion for both patient and physician.
-
Source: IMUC, OxBridge Research, Daily Stock Deal, OTCking
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
Disclaimer/Disclosure: we receive compensation from companies we feature on our websites/blogs/emails/newsletters or comment on social media/FB/TWTR etc. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.

Petro River Oil, PTRC, Profile, Summary

Petro River Oil | PTRC | Profile | Summary
Petro is an emerging oil and gas producer focusing on assets in the Southeast Kansas region of the Mississippi Lime. Petro owns approximately 115,000 gross acres (85,000 net acres) with five producing oil and gas wells, in which Petro owns a 50% working interest and 40% net revenue interest.
Petro River’s activities are focused on its oil properties in the Mississippi Lime play in eastern Kansas. The Company has accumulated close to 100,000 acres with an extensive inventory of low cost drilling opportunities with potential for high returns. At current commodity prices, Mississippi Lime wells are averaging a 80% rate of return. Petro River Oil also has significant acreage and oil reserves in Missouri.
Source: Petro River, OxBridge Research, Daily Stock Deal, OTCking
Don't miss the NEXT premium Alert! Sign-up, Get Alerts, MakeMoney!®
Disclaimer/Disclosure: we received or expecting compensation from the featured company. Our firm, principals and staff may own/buy/sell/trade stock/securities of this company. Always Read the full Disclosure/Disclaimer. Thanks.
- Dialogic, DLGC, Profile, Summary
- Green Earth Technologies, GETG, Profile, Summary
- OxBridge Research initiates coverage of Teryl Resources,TRC.V, with a price target of 14 cents.
- Titan Pharmaceuticals, TTNP, Profile, Summary
- $20,000 in just 5 months, on a thousand shares, MAKO made members rich! Deep insight, Real Results revealed!


 Penny Stock Monster
Penny Stock Monster Feed Entries
Feed Entries
